It’s surprising to learn that, no matter how bad your radiator is, you can clean your vehicle radiator with baking soda, it can clean clogged pores, corroded and rusty radiator transform into really clean and smooth.
It’s an old technique of flushing and cleaning out radiator in a cheap way, very effective in case, if you don’t have any dedicated cleaning chemical for radiator.
If you think it could hurt your vehicle’s radiator, don’t hesitate to use it, it’s really safe and sound and possibly the safest, for instance, in some conditions such as you couldn’t find or purchase special chemicals.
Before you step into how to clean a radiator with baking soda, you need to learn what baking soda is and why it is a useful cleaning solution and remember to add vinegar with baking soda as both create a chemical reaction that involves this.
What happens when you mix baking soda and vinegar?
Baking soda and vinegar are two strong products people love to use for cleaning. Each have their own individual sanitizing perks, according to Brian Sansoni of the American Cleaning Institute, an expert on all things cleaning. “Baking soda is a natural deodorizer and a fine abrasive; that’s good for odor absorption and scrubbing. Vinegar is especially great at lifting hard water stains. When you combine baking soda and vinegar, you mix an acid (vinegar) and a base (baking soda) which creates salty water and carbon dioxide gas. The reaction has an immediate ‘clean’ look, but when you look deeper you realize that you’re left with saltwater. The agitation of the fizzy reaction itself can be useful to physically break up and carry away dirt.
Chemical reaction of Baking soda and Vinegar
Baking soda is sodium bicarbonate: each molecule of baking soda contains a sodium atom, a hydrogen atom, an oxygen atom, and a carbon dioxide molecule.
Vinegar contains acetic acid, each molecule of which contains a hydrogen atom, and an acetate ion.
When combined, the hydrogen atom in the acetic acid meets up with the hydrogen and oxygen atoms in the baking soda to form a molecule of water, while the acetate ion grabs onto the sodium atom and forms a salt, sodium acetate. The carbon dioxide molecule, free of its other chemical bonds, can now escape, and bubbles forth as a gas.
More Car Maintenance: IS A BRAKE FLUID FLUSH NECESSARY?
Process of How to Clean Radiator with Baking Soda?
The amount of baking soda and vinegar remains equal to 6 tablespoons each, which will provide the best outcome.
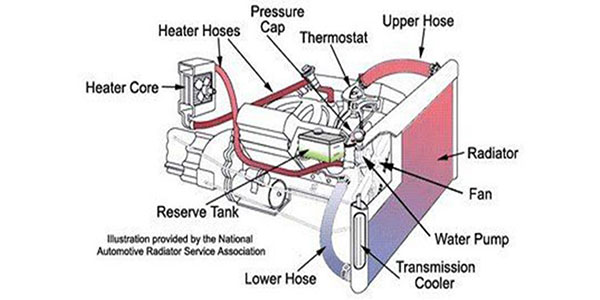
- Before doing so, Remove the thermostat valve, baking soda can stuck into a thermostat valve that could cause issues with controlling engine temperature, if you can’t take it out of the valve then leave this method and use dedicated chemicals for cleaning.
- In a radiator tank, Apply 6 Tablespoons of baking soda and vinegar in already filled water (do not put soda in the coolant, flush out the coolant, and water instead).
- Now you’ll see the bubbles and foamy reaction as gas.
- Close the radiator cap and turn the engine on and wait until the temperature of your car engine reaches its optimum position (gauge reach at a center stage point).
- After Achieving Optimum Temperature, turn off the engine and remove the cap, and then flush the radiator tank by removing the water pipe from the main lower hose.
- Fill the water again without placing it back on the lower hose pipe, it allows the radiator to flush it out of the radiator and clean all the rusty and other debris.
- Repeat this watering cycle again and again before you see clean water flowing out of a radiator.
- In the last Reverse method use, Put a garden hose on a heater hose/ Upper hose going into a heater core and push water through the heater core under pressure back into the engine and out through where the line was connected to the pump.
More Car Maintenance: Why Is My Car Air Conditioner Not Blowing Cold Air?
More Car Maintenance: Signs You Need a Coolant Flush
For more in detail here is a Video for better understanding of How to Clean a Radiator with Baking Soda